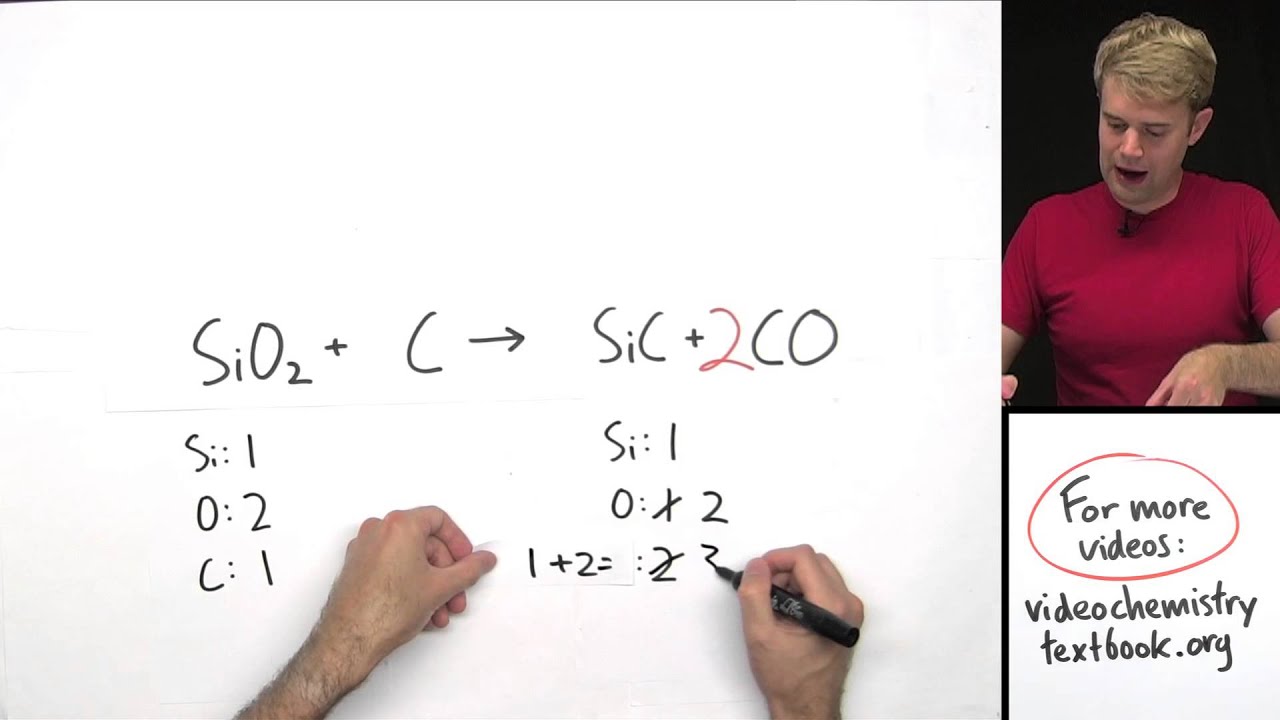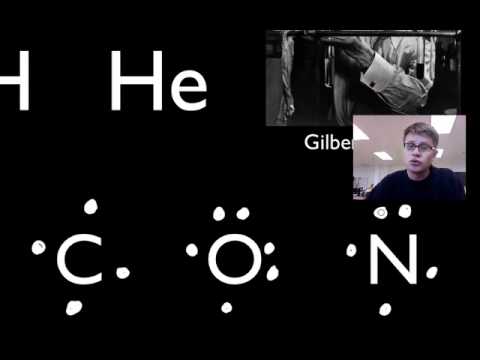Senior Secondary / High School
Sub Category
This animation shows the structure of matter at smaller and smaller scales. Zooming into a human hair, we p**** through hair cells, fibril structures, keratin molecules, Carbon atoms, nuclei, neutrons, protons, and finally quarks.
The Standard Model explains how the basic building blocks of matter interact, governed by four fundamental forces. Find out more: http://home.cern/…/physi…/standard-model
Produced by Daniel Dominguez/CERN
Copyright © CERN
Thanks to Google for sponsoring a portion of this video!
Support MinutePhysics on Patreon: http://www.patreon.com/minutephysics
This video is about using Bohmian trajectories to visualize the wavefunctions of hydrogen orbitals, rendered in 3D using custom python code in Blender.
REFERENCES
A Suggested Interpretation of the Quantum Theory in Terms of "Hidden" Variables. I
David Bohm, Physical Review, Vol 85 No. 2, January 15, 1952
Speakable and Unspeakable in Quantum Mechanics
J. S. Bell
Trajectory construction of Dirac evolution
Peter Holland
The de Broglie-Bohm Causal Interpretation of Quantum Mechanics and its Application to some Simple Systems by Caroline Colijn
Bohmian Trajectories as the Foundation of Quantum Mechanics
http://arxiv.org/abs/0912.2666v1
The Pilot-Wave Perspective on Quantum Scattering and Tunneling
http://arxiv.org/abs/1210.7265v2
A Quantum Potential Description of One-Dimensional Time-Dependent Scattering From Square Barriers and Square Wells
Dewdney, Foundations of Physics, VoL 12, No. 1, 1982
Link to Patreon Supporters: http://www.minutephysics.com/supporters/
MinutePhysics is on twitter - @minutephysics
And facebook - http://facebook.com/minutephysics
Minute Physics provides an energetic and entertaining view of old and new problems in physics -- all in a minute!
Created by Henry Reich
Here's how to write formulas for binary ionic compounds. We'll see how you have to balance the charges of the two ions so they cancel each other out.
Equation balancing will make sense! Here, we will do a bunch of practice problems for balancing chemical equations. We'll see the process or trial and error and the steps that you have to go through to balance chemical equations. You start by keeping track of the number of atoms on both sides of the equation, and then you add coefficients to one or more of the elements and compounds to make the number of atoms equal.
How to balance chemical equations. We'll start out with examples that show the concepts behind balancing chemical equations. We will start with a word equation, and then write a chemical equations, and then visualize the atoms and molecules and how they change. To figure out if the equations is balanced, we look at the number and type of atoms on each side of the arrow. If the number and type of atom is not the same on both sides, the equation in unbalanced. We need to change the number of one or more of the compounds in order to get the atoms to balance. We do this by placing coefficients (numbers) in front of each of the compounds. When balancing equations, you cannot ever change the subscripts of a compound.
Mr. Andersen describes the major groups on the periodic table.
Intro Music Atribution
Title: I4dsong_loop_main.wav
Artist: CosmicD
Link to sound: http://www.freesound.org/peopl....e/CosmicD/sounds/725
Creative Commons Atribution License
Hank gives us a tour of the most important table ever, including the life story of the obsessive man who championed it, Dmitri Mendeleev. The periodic table of elements is a concise, information-dense catalog of all of the different sorts of atoms in the universe, and it has a wealth of information to tell us if we can learn to read it.
Watch this video in Spanish on our Crash Course en Español channel! https://youtu.be/AmOl0v_3jsc
Pssst... we made flashcards to help you review the content in this episode! Find them on the free Crash Course App!
Download it here for Apple Devices: https://apple.co/3d4eyZo
Download it here for Android Devices: https://bit.ly/2SrDulJ
Table of Contents
Dmitri Mendeleev - 0:45
Mendeleev's Organization of the Periodic Table - 2:31
Relationships in the Periodic Table - 5:03
Why Mendeleev Stood Out from his Colleagues - 7:09
How the Periodic Table Could be Improved - 8:28
More info about the cylindrical periodic table of elements: http://www.av8n.com/physics/periodic-table.htm
Crash Course is on Patreon! You can support us directly by signing up at http://www.patreon.com/crashcourse
Want to find Crash Course elsewhere on the internet?
Facebook - http://www.facebook.com/YouTubeCrashCourse
Twitter - http://www.twitter.com/TheCrashCourse
Instagram - https://www.instagram.com/thecrashcourse/
CC Kids: http://www.youtube.com/crashcoursekids
How to memorize the periodic table 10X faster - Video 1. Start with the first 20 elements at https://www.memorize.academy/first-20-elements and then go for the entire periodic table of 118 elements at https://www.memorize.academy/m....emorize-the-periodic
How do you memorize the periodic table in the fastest and easiest way possible? You use the natural power of your visual memory.
We offer an easy way to memorize the periodic table. Memorization of the periodic table with our innovative animated video series takes just hours, so memorize the elements now!
Most people only know the typical techniques to memorize using your verbal memory – acronyms, acrostics, rhymes, ****ociations and songs. Those techniques can be great for remembering small amounts of information, but they don’t take advantage of the dramatic improvements to your recall when you activate your visual memory.
We’ll begin by picturing a typical poster or chart of the periodic table. There are many small, colorful squares, each with a name, number and symbol of an element, and together they create a large irregular shape.
This image will act as an anchor in your memory, holding down the chain of images which link together all the elements.
Now we’ll take that colorful poster and attach it to the first element. Picture that poster of the periodic table and imagine it’s wrapped around a water hydrant.
Why a water hydrant?
1. Hydrogen
The 1st element in the periodic table is Hydrogen. Hydrogen sounds similar to hydrant and that’s how you’ll be reminded of it. Picture a water hydrant you see on the sidewalk. It’s short, stubby, red, and looks strong. The hydrant is like a little man with a small hat on top and stubby arms sticking out the side.
Imagine that hydrant with the chart of the periodic table wrapped around it. When you think of the chart of the periodic table, you’ll picture it wrapped around a water hydrant. Because hydrant sounds similar to hydrogen, you’ll know the 1st element in the table is Hydrogen.
2. Helium
The 2nd element is Helium. If you’re like me, when you think of Helium, you automatically think of a helium balloon. When you let it go, it’s the type that floats up into the sky. Now imagine an enormous helium balloon. Make it the size of a car and picture it attached to the water hydrant. Because the helium balloon is so big and has so much lifting power, it starts to lift the water hydrant up off the sidewalk. Together they slowly float up into the air and away into the sky. Now, when you visualize the helium balloon floating upwards, you’ll know the 2nd element is Helium.
3. Lithium
The 3rd element is Lithium. Lithium sounds a bit like “lithp”. People that have a lisp – a type of speech impediment – aren’t able to pronounce “lisp” and say “lithp”. Let’s pretend the large helium balloon has a lisp. It also has a small hole in it, causing the balloon to slowly deflate. Usually a balloon with a hole in it will make a slow “ssss” sound, but because this balloon has a lisp or “lithp”, it makes a “thhh” sound. Visualize the large balloon slowly deflating making a “thhh” sound. When you think of the balloon’s “lithp”, you’ll be reminded of the 3rd element, Lithium.
4. Beryllium
The 4th element is Beryllium. If you say Beryllium slowly, it sounds like “bee really yum”. Picture your slowly deflating balloon. Imagine an enormous bumble bee lands on the balloon. The bee is the size of a football and has bright yellow and black stripes and buzzes loudly. The bee licks the balloon to have a taste and says, “that’s really yum!” It really likes the taste of the balloon. When you picture the bee licking the balloon, you’ll think, “bee really yum”, and be reminded of the 4th element, Beryllium.
5. Boron
The 5th element is Boron. We can break up the word Boron into “bore” and “on”. The word “bore” can mean to drill a hole. Picture now the bee, after tasting the balloon. It uses its stinger, pierces the balloon and starts to spin around in a drilling motion. The bee has landed on the balloon, tasted it, and now it’s started to “bore on” the balloon. When you picture the bee begin to bore on the balloon, you’ll remember the 5th element, Boron.
Check out our UPDATED version which has all the NEW ELEMENTS here: https://youtu.be/rz4Dd1I_fX0
The END OF THE UNIVERSE Song: https://youtu.be/o6UPfdhOHIY
Download on ITUNES: http://bit.ly/12AeW99
DOWNLOAD ON BANDCAMP: http://bit.ly/111Kssd (instrumental available)
Get the AsapSCIENCE Book! http://asapscience.com/book
FOLLOW US!
Instagram and Twitter: @whalewatchmeplz and @mitchellmoffit
Clickable: http://bit.ly/16F1jeC and http://bit.ly/15J7ube
AsapINSTAGRAM: https://instagram.com/asapscience/
Facebook: http://facebook.com/AsapSCIENCE
Twitter: http://twitter.com/AsapSCIENCE
Tumblr: http://asapscience.tumblr.com
Vine: Search "AsapSCIENCE" on vine!
SNAPCHAT 'whalewatchmeplz' and 'pixelmitch'
Send us stuff!
ASAPSCIENCE INC.
P.O. Box 93, Toronto P
Toronto, ON, M5S2S6
------------------------------------------
Written, Directed, Produced, Edited and Sung by Mitchell Moffit.
Based on the "Can-Can" music, by Offenbach.
LYRICS:
There's Hydrogen and Helium
Then Lithium, Beryllium
Boron, Carbon everywhere
Nitrogen all through the air
With Oxygen so you can breathe
And Fluorine for your pretty teeth
Neon to light up the signs
Sodium for salty times
Magnesium, Aluminium, Silicon
Phosphorus, then Sulfur, Chlorine and Argon
Pot****ium, and Calcium so you'll grow strong
Scandium, Titanium, Vanadium and Chromium and Manganese
CHORUS
This is the Periodic Table
Noble gas is stable
Halogens and Alkali react agressively
Each period will see new outer shells
While electrons are added moving to the right
Iron is the 26th
Then Cobalt, Nickel coins you get
Copper, Zinc and Gallium
Germanium and Arsenic
Selenium and Bromine film
While Krypton helps light up your room
Rubidium and Strontium then Yttrium, Zirconium
Niobium, Molybdenum, Technetium
Ruthenium, Rhodium, Palladium
Silver-ware then Cadmium and Indium
Tin-cans, Antimony then Tellurium and Iodine and Xenon and then Caesium and...
Barium is 56 and this is where the table splits
Where Lanthanides have just begun
Lanthanum, Cerium and Praseodymium
Neodymium's next too
Promethium, then 62's
Samarium, Europium, Gadolinium and Terbium
Dysprosium, Holmium, Erbium, Thulium
Ytterbium, Lutetium
Hafnium, Tantalum, Tungsten then we're on to
Rhenium, Osmium and Iridium
Platinum, Gold to make you rich till you grow old
Mercury to tell you when it's really cold
Thallium and Lead then Bismuth for your tummy
Polonium, Astatine would not be yummy
Radon, Francium will last a little time
Radium then Actinides at 89
REPEAT CHORUS
Actinium, Thorium, Protactinium
Uranium, Neptunium, Plutonium
Americium, Curium, Berkelium
Californium, Einsteinium, Fermium
Mendelevium, Nobelium, Lawrencium
Rutherfordium, Dubnium, Seaborgium
Bohrium, H****ium then Meitnerium
Darmstadtium, Roentgenium, Copernicium
Ununtrium, Flerovium
Ununpentium, Livermorium
Ununseptium, Ununoctium
And then we're done!!
This week Reactions is bringing you some chemistry life hacks! Science can help you cure bitter coffee, ripen your bananas quickly, breathe life back into stale cookies, and remove rust from cast iron.
0:16 - Cure bitter coffee
0:54 - Ripen your bananas overnight
1:30 - Bring life back into stale cookies
2:03 - Remove rust from cast iron
Check out our debut Reactions video to find easy chemistry-fueled solutions for everyday dilemmas, plus more useful tips to improve your life dramatically (OK, maybe just a little bit).
Find us on all these places:
Subscribe! http://bit.ly/ACSReactions
Facebook! http://facebook.com/ACSReactions
Twitter! http://twitter.com/ACSReactions
Video directed and produced by Kirk Zamieroski
Co-directed by Adam Dylewski
Produced by the American Chemical Society
Roberto Daglio - Bibossa - Provided by Jamendo
Mr. Andersen explains the basics of balancing chemical equations. A visual guide shows you how to change coefficients to balance the atoms in reactants and products.
Intro Music Atribution
Title: I4dsong_loop_main.wav
Artist: CosmicD
Link to sound: http://www.freesound.org/peopl....e/CosmicD/sounds/725
Creative Commons Atribution License
Mr. Andersen shows you how write the chemical formula for chemical names.
Intro Music Atribution
Title: I4dsong_loop_main.wav
Artist: CosmicD
Link to sound: http://www.freesound.org/peopl....e/CosmicD/sounds/725
Creative Commons Atribution License
Mr. Andersen shows you how to name covalent and ionic compounds.
Intro Music Atribution
Title: I4dsong_loop_main.wav
Artist: CosmicD
Link to sound: http://www.freesound.org/peopl....e/CosmicD/sounds/725
Creative Commons Atribution License
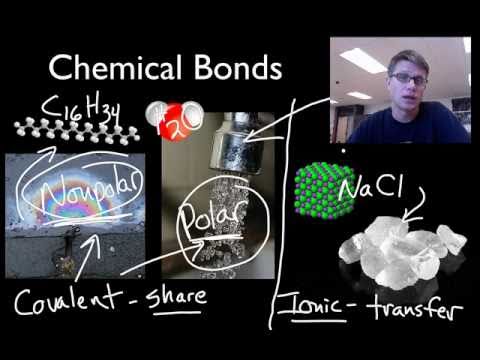
Mr. Andersen shows you how to determine if a bond is nonpolar covalent, polar covalent, or ionc.
Intro Music Atribution
Title: I4dsong_loop_main.wav
Artist: CosmicD
Link to sound: http://www.freesound.org/peopl....e/CosmicD/sounds/725
Creative Commons Atribution License
020 - Ionic Bonding
In this video Paul Andersen explains how ionic solids form when cations and anions are attracted. When atoms lose or gain electrons they form ions. The strength of the attraction between ions is based on the amount of charge and the distance between the ions.
Music Attribution
Title: String Theory
Artist: Herman Jolly
http://sunsetvalley.bandcamp.c....om/track/string-theo
All of the images are licensed under creative commons and public domain licensing:
"File:Cat November 2010-1a.jpg." Wikipedia, the Free Encyclopedia, July 19, 2013. http://en.wikipedia.org/w/index.php?title=File:Cat_November_2010-1a.jpg&oldid=517556234.
"File:Chloride-ion-3D-vdW.png." Wikipedia, the Free Encyclopedia. Accessed August 12, 2013. http://en.wikipedia.org/wiki/F....ile:Chloride-ion-3D-
"File:CoulombsLaw.svg." Wikipedia, the Free Encyclopedia. Accessed August 12, 2013. http://en.wikipedia.org/wiki/File:CoulombsLaw.svg.
"File:NaCl Polyhedra.png." Wikipedia, the Free Encyclopedia. Accessed August 12, 2013. http://en.wikipedia.org/wiki/F....ile:NaCl_polyhedra.p
"File:NaCl.png." Wikipedia, the Free Encyclopedia. Accessed August 12, 2013. http://en.wikipedia.org/wiki/File:NaCl.png.
"File:Periodic Trends.svg." Wikipedia, the Free Encyclopedia. Accessed August 12, 2013. http://en.wikipedia.org/wiki/F....ile:Periodic_trends.
"File:Sodium-bromide-3D-ionic.png." Wikipedia, the Free Encyclopedia. Accessed August 12, 2013. http://en.wikipedia.org/wiki/F....ile:Sodium-bromide-3
"File:Sodium-fluoride-3D-ionic.png." Wikipedia, the Free Encyclopedia. Accessed August 12, 2013. http://en.wikipedia.org/wiki/F....ile:Sodium-fluoride-
"File:Sodium-iodide-3D-ionic.png." Wikipedia, the Free Encyclopedia. Accessed August 12, 2013. http://en.wikipedia.org/wiki/F....ile:Sodium-iodide-3D
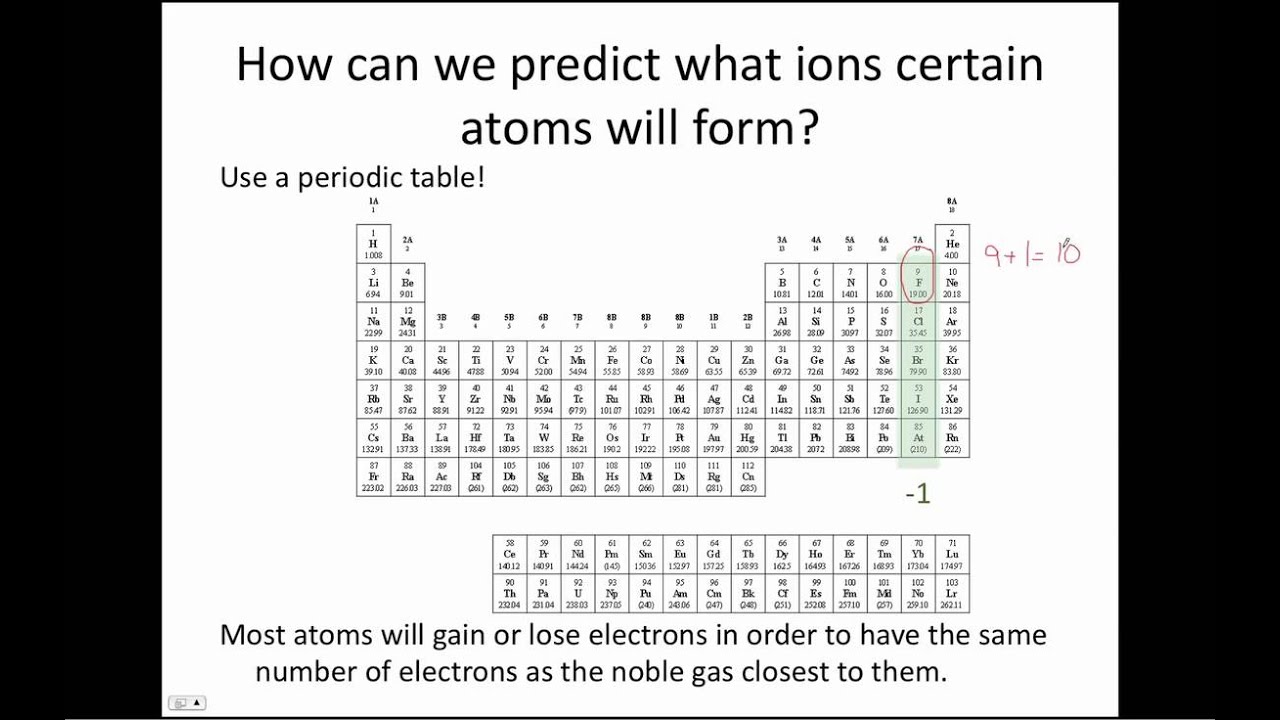
Ionic Compounds: Predicting Ion Charges and Empirical Formulas
This tutorial covers how to predict the charges of commonly formed ions using the periodic table and also how to determine empirical formulas for ionic compounds.
https://www.thechemsolution.com
Learn the basics about Ionic Compounds, how they are formed and what their properties are.
At Fuse School, teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT. Our OER are available free of charge to anyone. Make sure to subscribe - we are going to create 3000 more!
Fuse School is currently running the Chemistry Journey project - a Chemistry Education project by The Fuse School sponsored by Fuse. These videos can be used in a flipped cl****room model or as a revision aid. Find our other Chemistry videos here:
https://www.youtube.com/playli....st?list=PLW0gavSzhMl
Be sure to follow our social media for the latest videos and information!
Twitter: https://twitter.com/fuseschool
Facebook: https://www.facebook.com/fuseschool
Google+: http://www.gplus.to/FuseSchool
Youtube: http://www.youtube.com/virtualschooluk
Email: info@fuseschool.org
Website: www.fuseschool.org
This video is distributed under a Creative Commons License:
Attribution-NonCommercial-NoDerivs CC BY-NC-ND
In this video you'll learn the basics about Ionic Bonds.
SUPPORT US ON PATREON
https://www.patreon.com/fuseschool
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
VISIT us at [a]www.fuseschool.org%2C[/a] where all of our videos are carefully organised into topics and specific orders, and to see what else we have on offer. Comment, like and share with other learners. You can both ask and answer questions, and teachers will get back to you.
These videos can be used in a flipped cl****room model or as a revision aid.
Find all of our Chemistry videos here: https://www.youtube.com/playli....st?list=PLW0gavSzhMl
Find all of our Biology videos here: https://www.youtube.com/playli....st?list=PLW0gavSzhMl
Find all of our Physics videos here: https://www.youtube.com/playli....st?list=PLW0gavSzhMl
Find all of our Maths videos here: https://www.youtube.com/playli....st?list=PLW0gavSzhMl
Instagram: https://www.instagram.com/fuseschool/
Facebook: https://www.facebook.com/fuseschool/
Twitter: https://twitter.com/fuseSchool
Access a deeper Learning Experience in the FuseSchool platform and app: www.fuseschool.org
Follow us: http://www.youtube.com/fuseschool
Befriend us: http://www.facebook.com/fuseschool
This is an Open Educational Resource. If you would like to use the video, please contact us: info@fuseschool.org
What Are Ions | Properties of Matter | Chemistry | FuseSchool
What is an ion? What role does it have to play in the structure of atoms? Watch this video to find out!
JOIN US ON PATREON
https://www.patreon.com/fuseschool
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
VISIT us at [a]www.fuseschool.org%2C[/a] where all of our videos are carefully organised into topics and specific orders, and to see what else we have on offer. Comment, like and share with other learners. You can both ask and answer questions, and teachers will get back to you.
These videos can be used in a flipped cl****room model or as a revision aid.
Find all of our Chemistry videos here: https://www.youtube.com/playli....st?list=PLW0gavSzhMl
Find all of our Biology videos here: https://www.youtube.com/playli....st?list=PLW0gavSzhMl
Find all of our Physics videos here: https://www.youtube.com/playli....st?list=PLW0gavSzhMl
Find all of our Maths videos here: https://www.youtube.com/playli....st?list=PLW0gavSzhMl
Instagram: https://www.instagram.com/fuseschool/
Facebook: https://www.facebook.com/fuseschool/
Twitter: https://twitter.com/fuseSchool
Access a deeper Learning Experience in the FuseSchool platform and app: www.fuseschool.org
Follow us: http://www.youtube.com/fuseschool
Befriend us: http://www.facebook.com/fuseschool
This is an Open Educational Resource. If you would like to use the video, please contact us: info@fuseschool.org
Mr. Andersen shows you how to draw Lewis Dot Diagrams for atoms and simple molecules.
Intro Music Atribution
Title: I4dsong_loop_main.wav
Artist: CosmicD
Link to sound: http://www.freesound.org/peopl....e/CosmicD/sounds/725
Creative Commons Atribution License